#1 LifeVac – The Proven, Non-Invasive Rescue Device
EDITOR'S CHOICE
Based on real-world uses & clinical studies
Its patented one-way valve is a critical safety feature that prevents air from pushing an object further down during use. LifeVac is a medical device registered with the USA's FDA, and it's the only device in its class with independent, peer-reviewed studies on its safety and effectiveness.
For its proven track record, superior safety design, and backing by medical professionals, LifeVac is the clear choice for family safety.
Pros
- Highest Documented Success Rate: Demonstrated a 99% success rate in manikin trials, significantly outperforming both Dechoker (74%) and traditional abdominal thrusts (71%).
- Safe & Non-Invasive Design: The patented one-way valve ensures no air is pushed into the victim, a critical failure point in dangerous counterfeit devices.
- Backed by Medical Professionals: Trusted and placed in thousands of hospitals, schools, and emergency service vehicles worldwide.
- Clinically Studied: The most studied device in its class, with multiple peer-reviewed publications on its efficacy and safety.
- No Prescription Needed: Simple and intuitive design allows anyone to act confidently in an emergency.
- Free Replacement Policy: If you use your LifeVac to save a life, the company provides a free replacement, reinforcing their commitment to safety.
Cons
- Purchase From Official Sources Only: The FDA has warned about the risks of unapproved OTC anti-choking devices. To avoid dangerous counterfeits, it must be bought directly from LifeVac or authorized distributors.
- Does Not Replace First Aid Training: LifeVac is intended for use only after standard BLS choking protocols (back blows/abdominal thrusts) have failed.
#2 Dechoker – Airway Suction Device

Based on user reviews
While also registered with the FDA, the company received an official FDA Warning Letter in 2021 for significant quality control failures. Studies have also shown it to be less effective than LifeVac and documented a risk of oral injury.
Pros
- Recognizable Brand Name: One of the first devices on the market, giving it brand recognition.
- Age-Specific Models: Available in different sizes for toddlers, children, and adults.
- Compact and Portable: Easy to store for quick access.
Cons
- Risk of Oral Injury: The invasive tube has been documented to cause "gross injury to the tongue" and lacerations in a human cadaver study.
- Lower Efficacy in Studies: Achieved only a 74% success rate in manikin trials, which was not statistically superior to abdominal thrusts (71%).
- FDA Quality Control Warning: Received a formal FDA Warning Letter for multiple failures in manufacturing processes, design controls, and complaint investigations.
- Single-Use Design: The device is intended to be discarded after a single use, making it more costly for long-term preparedness.
#3 ArixMed – Anti-Choking Device

Based on online marketplace reviews
While some users report adequate suction, there are concerns about the long-term durability of the rubber, which can degrade over time, especially if stored in a high-temperature environment like a vehicle.
Pros
- Multiple Mask Sizes: Often includes masks for both children and adults in a single package.
- Portable Design: Generally lightweight and easy to store.
Cons
- Material Durability Concerns: Rubber components may degrade over time or in heat, potentially compromising the device’s seal and effectiveness in an emergency.
- Mixed Performance Reviews: User feedback is inconsistent, with some reporting difficulty achieving a proper seal to generate effective suction.
- No Clinical Validation: Lacks sufficient clinical evidence or FDA oversight to support its efficacy.
#4 SaveLix – Anti-Choking Device
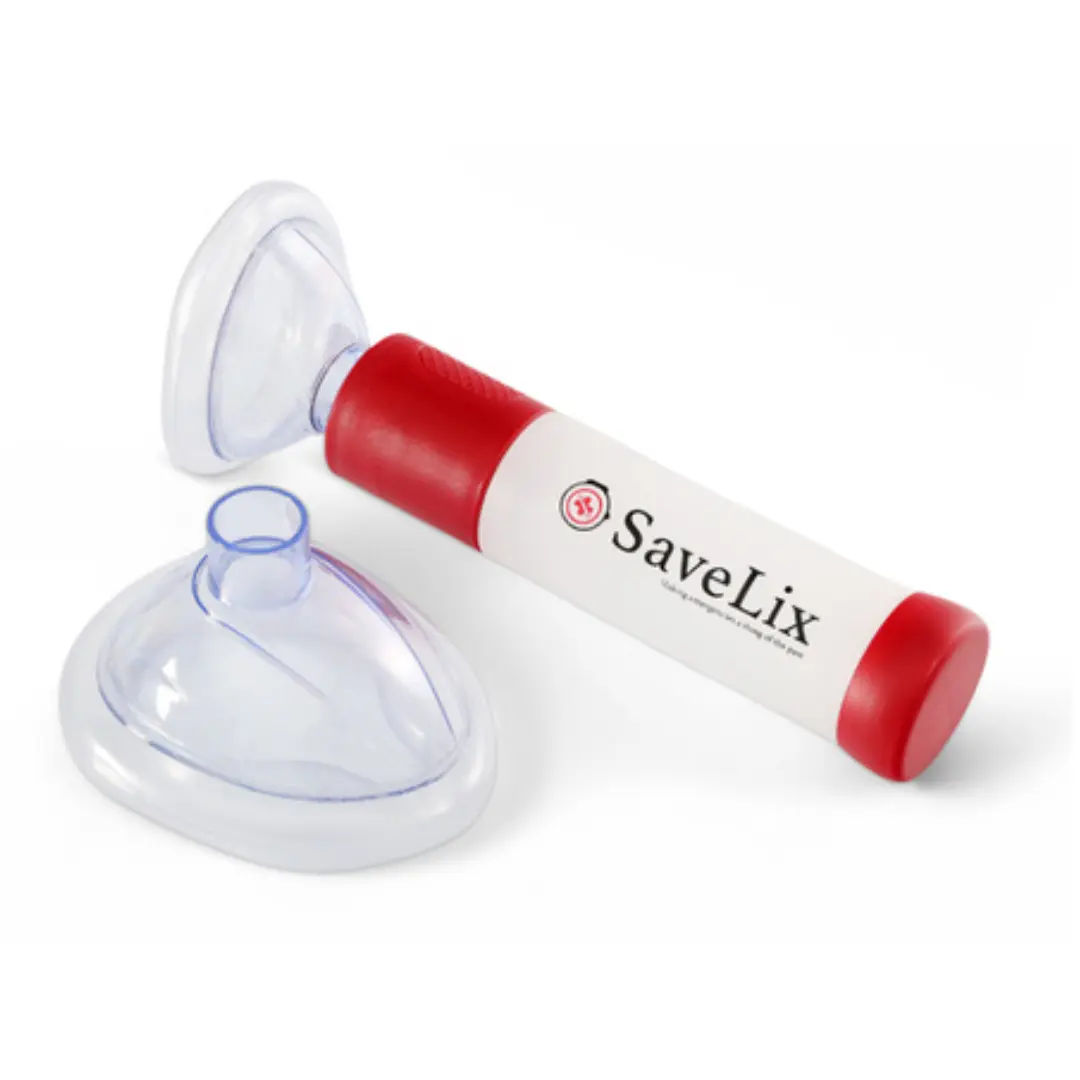
Based on online marketplace reviews
However, like other marketplace brands, it has a limited market presence and lacks extensive user reviews or credible clinical studies to verify its claims of safety and effectiveness.
Pros
- Simple Design: Aims to be straightforward and easy to understand.
Cons
- Limited Market Presence: Lacks widespread adoption and extensive user feedback compared to established brands.
- Unverified Efficacy: No independent clinical studies are available to validate its effectiveness or safety in real-world scenarios.
- Potential Counterfeit Risk: Falls into the category of unregulated devices the FDA has warned the public about.
#5 DANGER: AirwayClear™ & Other Counterfeits
DANGEROUS COUNTERFEIT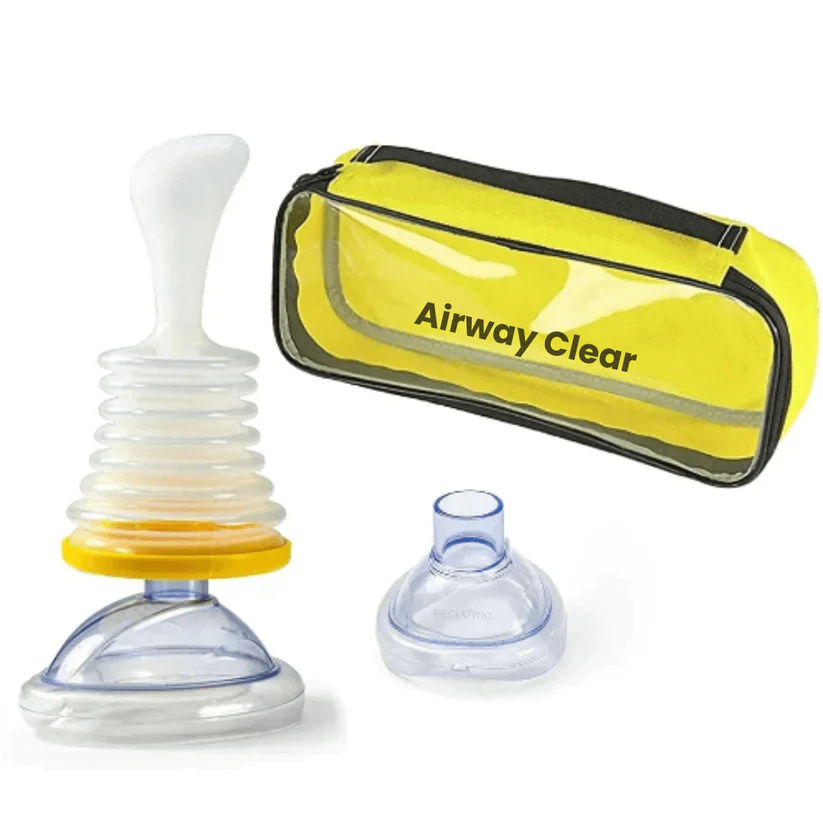
Based on FDA safety alerts
The USA's FDA has issued safety communications warning consumers about the risks of using such unapproved, over-the-counter devices. They are often made with cheap materials and may lack critical safety features.
Pros
- Lower Price Point: Their only appeal is a low price, which comes at the cost of safety and effectiveness.
Cons
- EXTREMELY DANGEROUS: These devices may lack a one-way safety valve. Pushing the plunger can force an obstruction deeper into the airway, making a choking situation fatal.
- Illegal and Unregulated: These are not registered with the FDA and do not meet any basic safety, quality, or efficacy standards.
- No Clinical Evidence: There are no studies supporting their use; on the contrary, their design is known to be dangerous.